- Home
- Company
- Products
- Status COVID-19/Flu A&B
- Status Flu A&B
- Status Strep A Flip Cassette Tests
- Indicaid COVID-19 Rapid Antigen Test
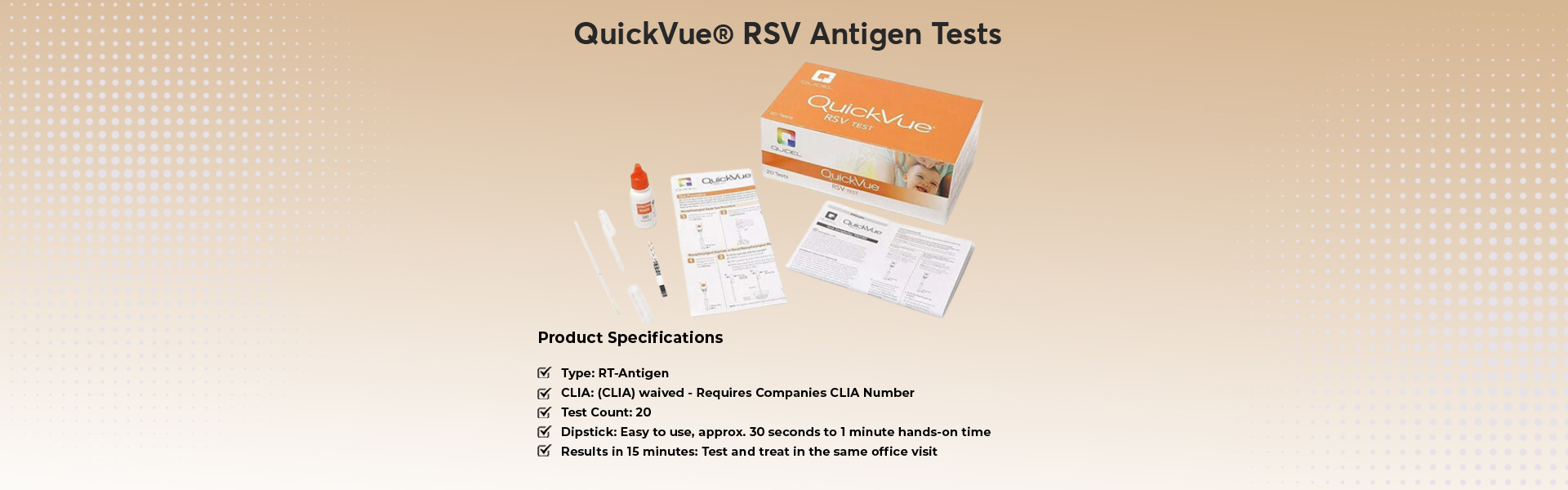
- QuickVue RSV Antigen Tests
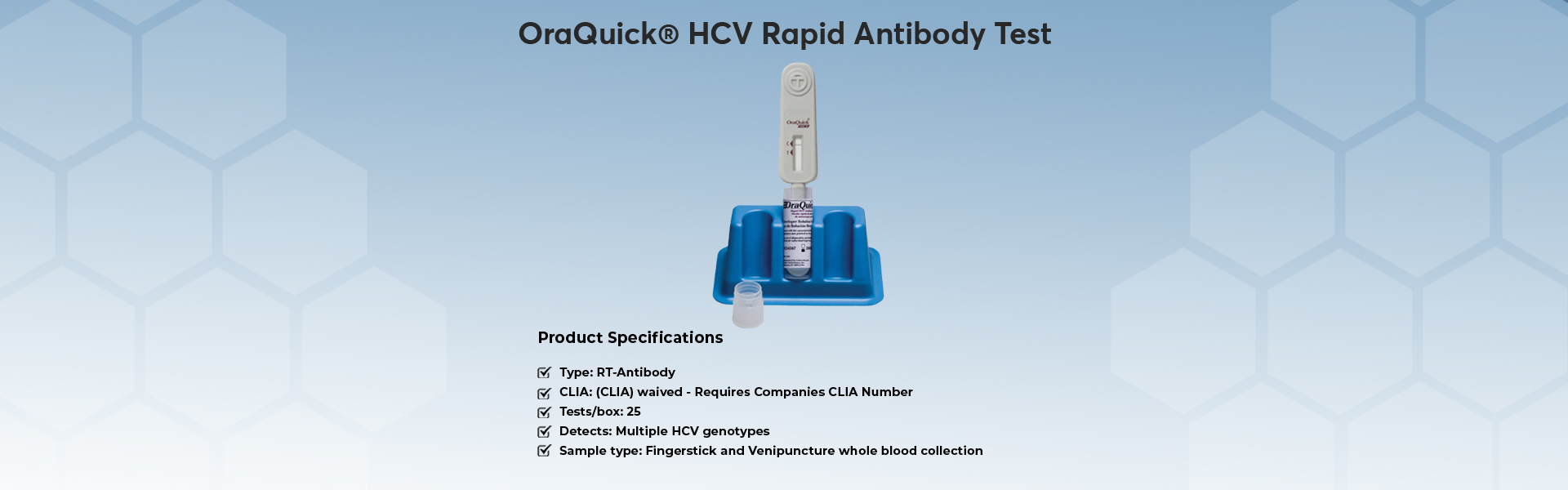
- OraQuick HCV Rapid Antibody Test
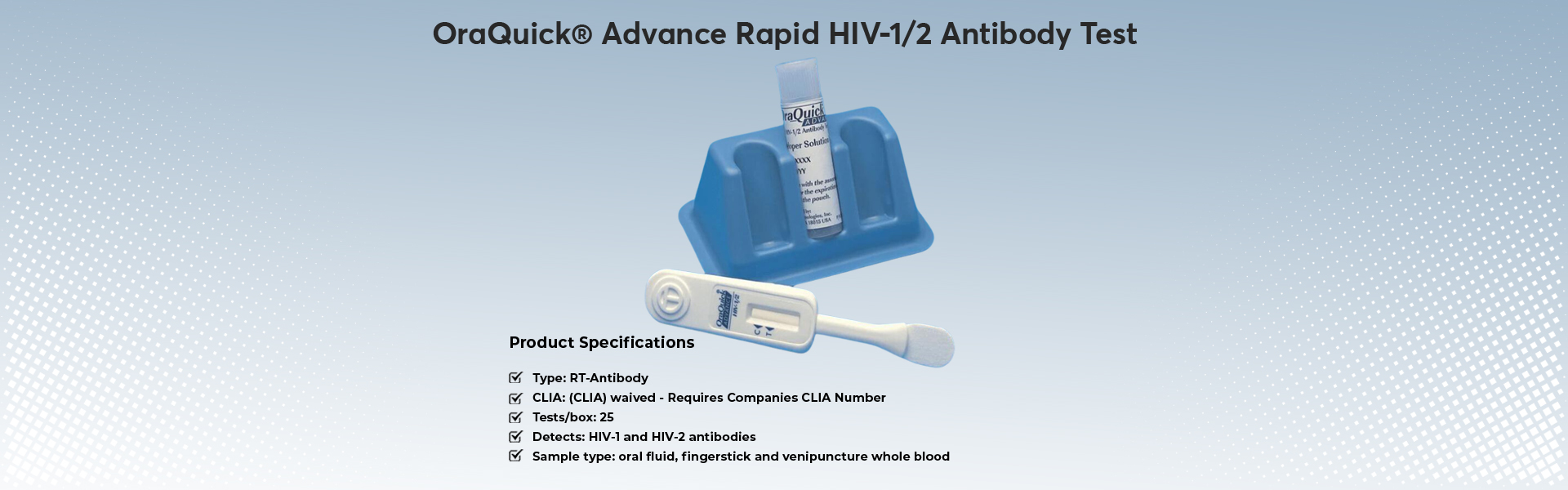
- OraQuick Advance Rapid HIV-1/2 Antibody Test
- DUKES BioFlexx Gloves 200 pack
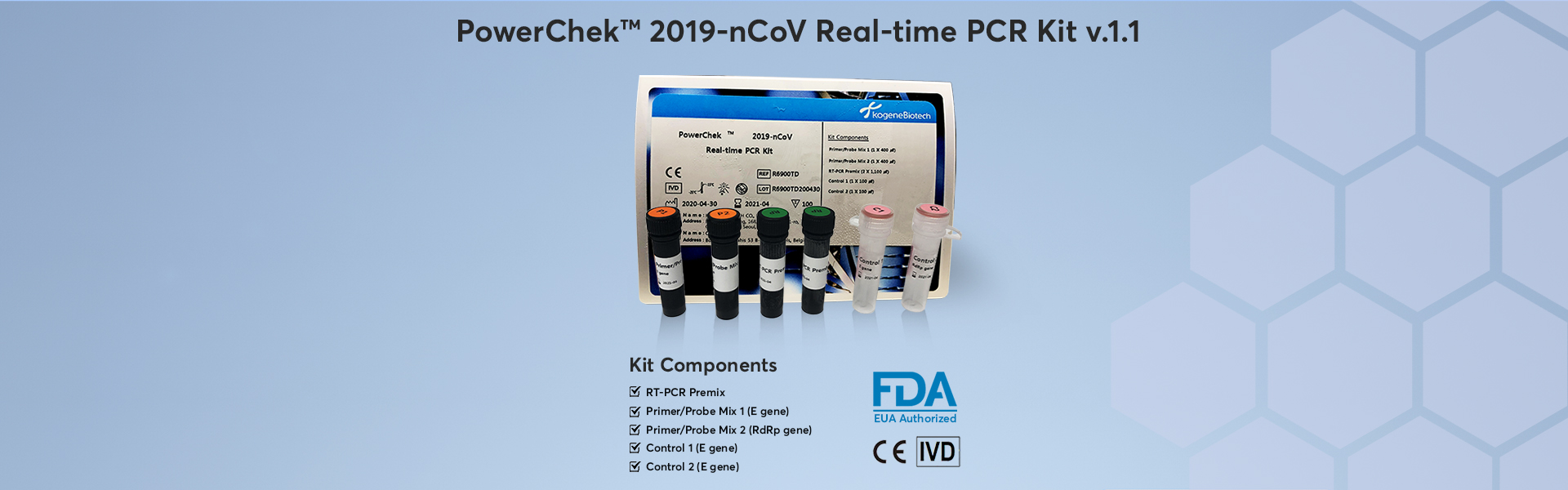
- PowerChek 2019-nCoV Real-time PCR Kit
- Specimen Collection and Transport Kit
- Contact Us



Explore our popular COVID-19 Test Kits
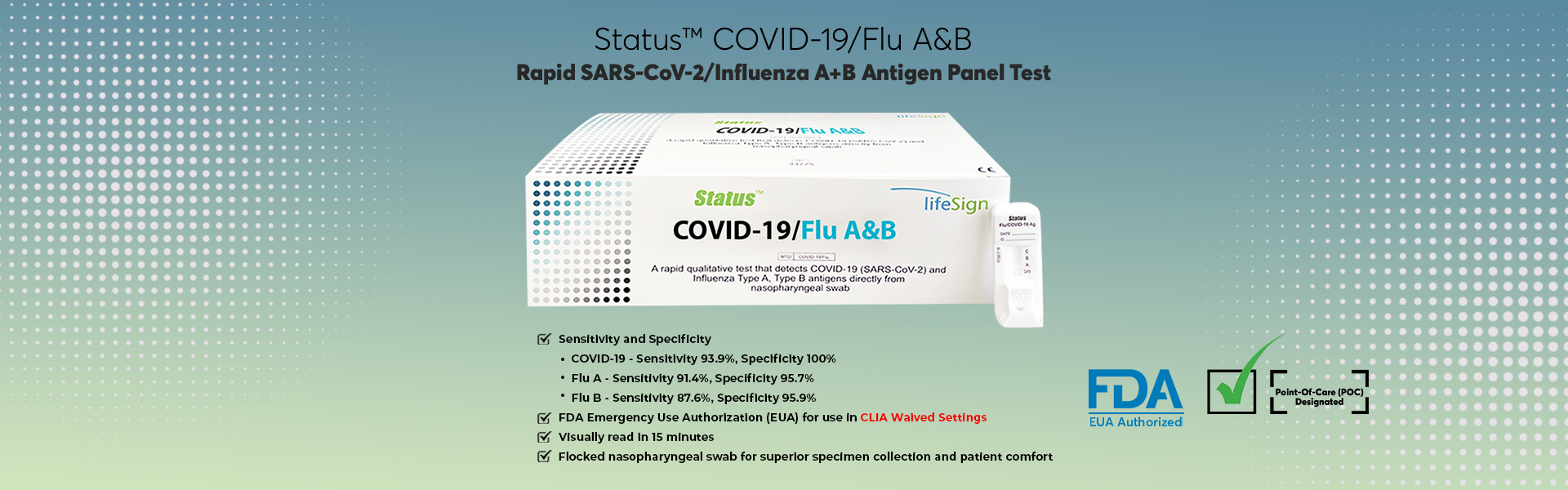
Rapid SARS-CoV-2/Influenza A+B Antigen Panel Test
Point-of-Care Rapid Test for three Viral Antigens from Just one swab sample
Results in about 15 Minutes — Quick and Reliable
FDA EUA authorized Antigen test
Read More …
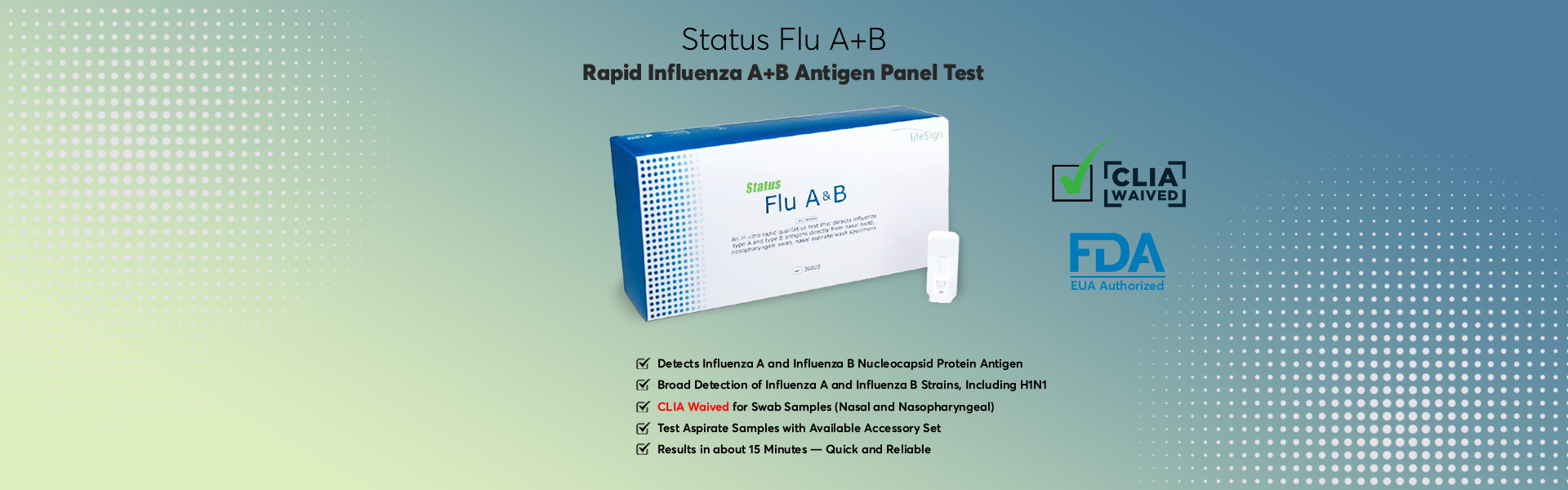
BioSign® Flu A+B – Rapid Influenza A+B Antigen Panel Test
Rapid, Point-of-Care Antigen Test
Results in about 15 Minutes
– Quick and Reliable
Detects Influenza A and Influenza B Nucleocapsid Protein Antigen
Read More …
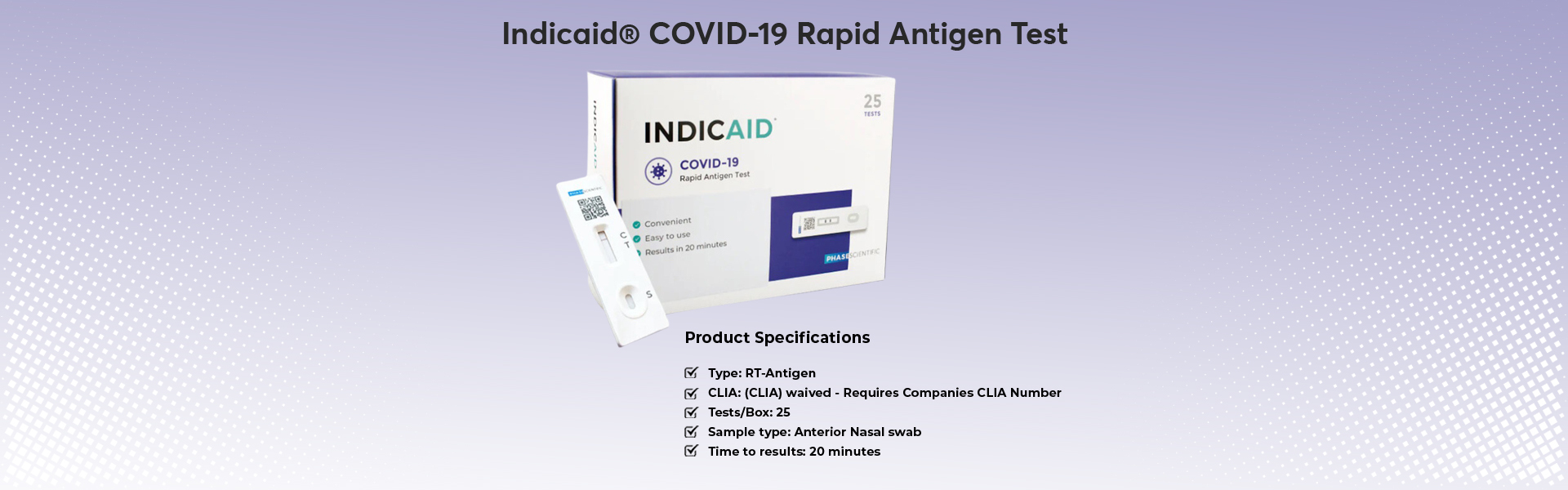
Type: RT-Antigen
Test Count: 25
A rapid immunochromatographic assay for the detection of group A streptococcal antigen directly from throat swab specimens. The test kit features patented single-use reagent capsules and has market leading
Read More …
The INDICAID® COVID-19 Rapid Antigen Test is a lateral flow immunoassay designed for the objective identification of SARS-CoV-2 antigens. Antigen tests are designed to detect proteins from the virus that causes COVID-19 through swab specimens taken from a patient’s nose.
Read More …
Test Count: 20
Simple 3-Step Testing Process
Simplified nasal swab sample collection
Single dipstick test
Approximately 30 seconds to 1 minute hands-on time
Results in 15 minutes or less; allowing to test and treat in the same office visit
Visible, two-color result
Read More …
Test Count: 1
The OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test is a single-use, qualitative immunoassay to detect antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) and Type 2 (HIV-2) in oral fluid, fingerstick whole blood, venipuncture whole blood and plasma specimens. Test is intended for use as a point
Read More …
The OraQuick® HCV test is the first FDA approved test for detecting HCV antibodies in finger-stick and venipuncture whole blood. The simple platform enables healthcare providers to deliver an accurate diagnosis in 20 minutes.
This is the initial laboratory test used for people with signs or symptoms, or who are at risk of having HCV infection.
Read More …
Count: 200
Biodegradable Powder-Free Examination Gloves, Non-sterile. Tested for use with Chemotherapy Drugs and Opioid Fentanyl Citrate.
Product Conformance:
This product meets current version of ASTM D6319, ASTM D6978, ASTM D5526, ASTM D5511 and is manufactured in accordance with a quality management that conforms
Read More …
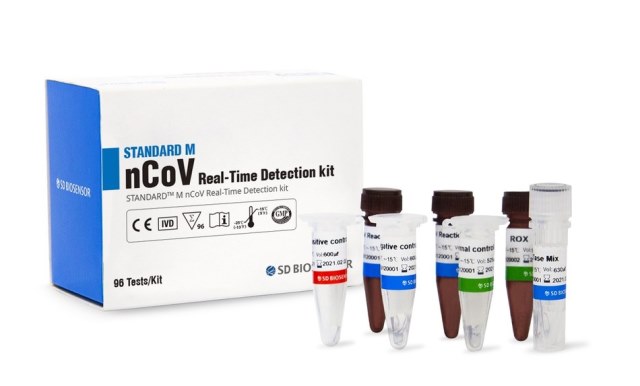
PowerChek 2019-nCoV Real-time PCR Kit provides the fast and accurate testing solution for Wuhan coronavirus, targeting he E gene for beta Coronavirus and the RdRp gene for COVID-19 specifically
Read More …
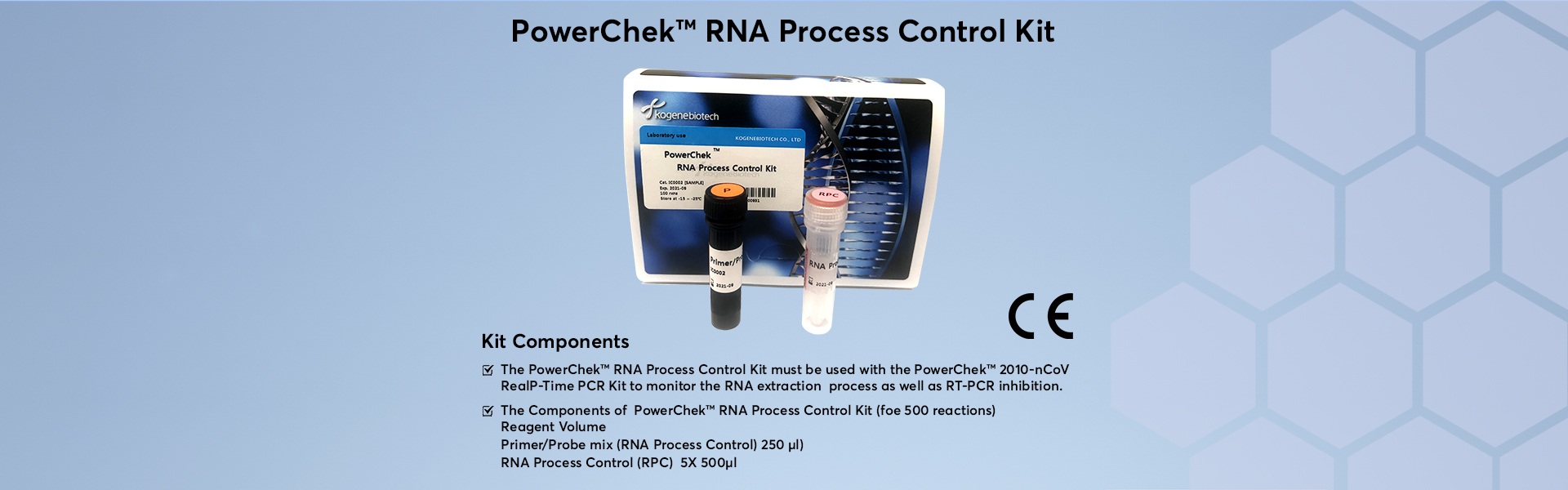
PowerChek RNA Process Control Kit must be used with the PowerChek 2019-nCoV Real-time PCR Kit to monitor the RNA extraction process as well as RT-PCR inhibition.
Read More …
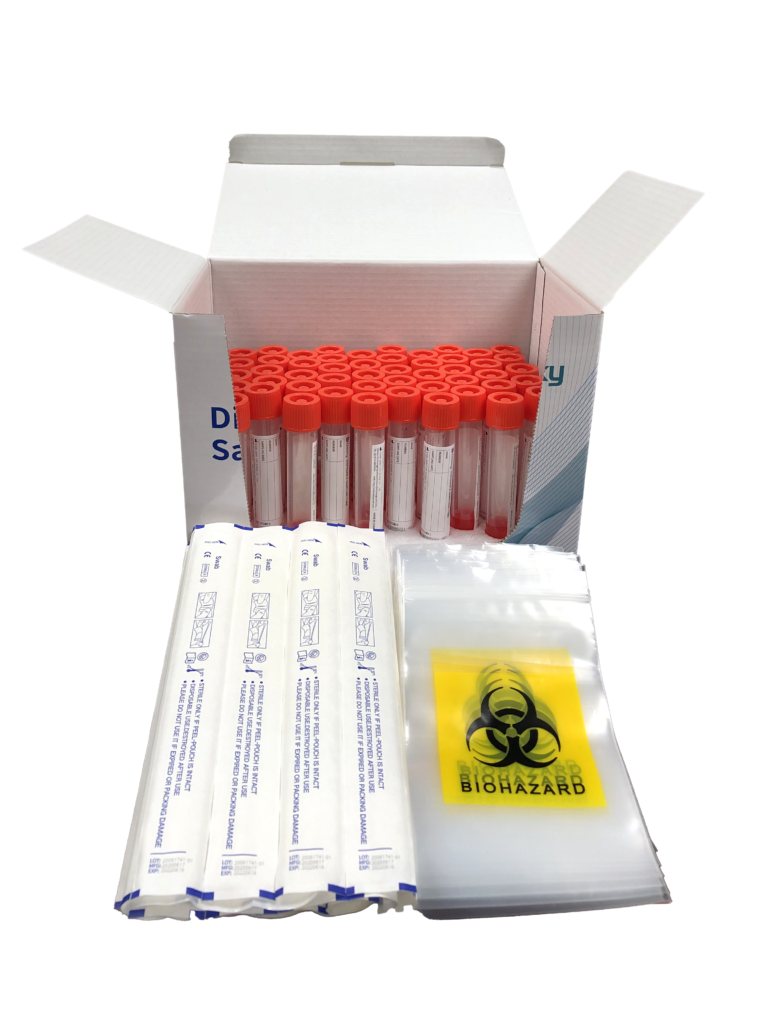
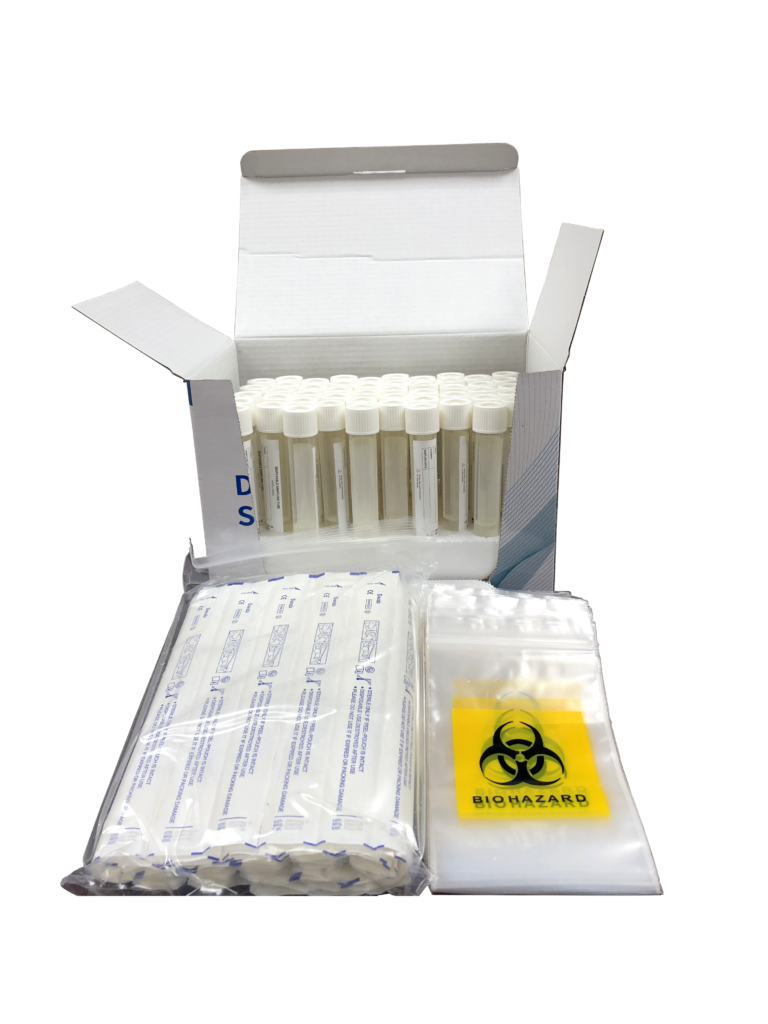
The product is used to collect high-quality DNA/RNA samples in the saliva. The collection process is painless and won’t cause any injury or discomfort to the human body. The collected samples can be used for various biological experiments and are widely used in the collection and preservation of specimens. Read More …
PowerChek RNA Process Control Kit must be used with the PowerChek 2019-nCoV Real-time PCR Kit to monitor the RNA extraction process as well as RT-PCR inhibition.
PowerChek 2019-nCoV Real-time PCR Kit provides the fast and accurate testing solution for Wuhan coronavirus, targeting he E gene for beta Coronavirus and the RdRp gene for COVID-19 specifically
- A simple protocol for isolation of viral RNA from any volume of a biological liquid, swabs (NPS) collected into a viral transport medium (VTM)
- Optional isolation and separation of RNA and DNA from the same sample
- Low cost
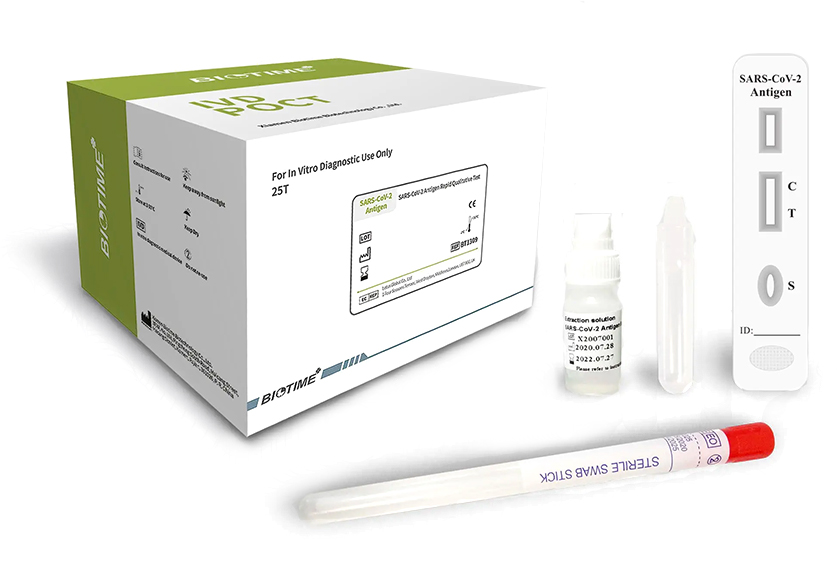
Our BioTime SARS-CoV-2 IgM/IgG Rapid Qualitative Test is a Highly Accurate with good Sensitivity & Specificity
The product is used to collect high-quality DNA/RNA samples in the saliva. The collection process is painless and won’t cause any injury or discomfort to the human body. The collected samples can be used for various biological experiments and are widely used in the collection and preservation of specimens. Read More …
Our SARS-CoV-2 Antigen Rapid Qualitative Test is a Highly Accurate with good Sensitivity & Specificity.